When they convince the immune system to work against the cancer the positive effects can last much longer than traditional chemotherapies. Nivolumab and ipilimumab represent a new treatment possibility shaping the options available to treat difficult diseases like mesothelioma.
Https Www Nice Org Uk Guidance Gid Ta10498 Documents Draft Scope Post Referral
An ongoing phase ii clinical trial for nivolumab opdivo and ipilimumab yervoy shows that the two immunotherapy drugs can slow the growth of malignant pleural mesothelioma after relapse.

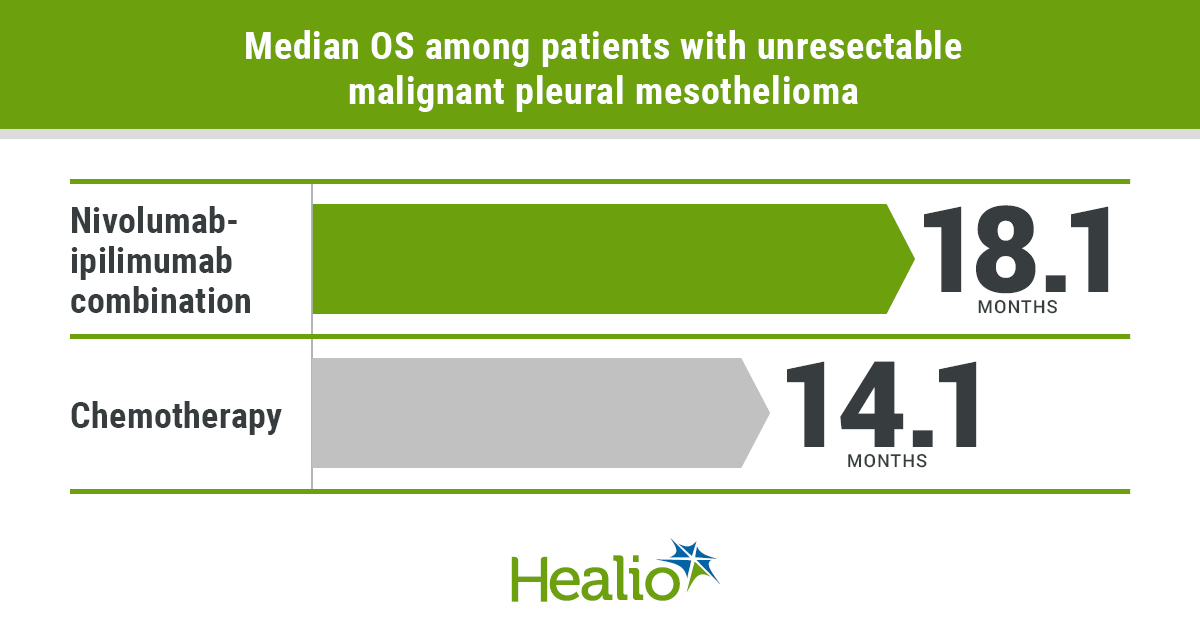
Mesothelioma nivolumab ipilimumab. Anti pd 1 nivolumab monotherapy or nivolumab plus anti ctla 4 ipilimumab combination therapy both showed promising activity in relapsed patients with malignant pleural mesothelioma without unexpected toxicity. Nivolumab plus ipilimumab significantly improved overall survival versus chemotherapy in the first line treatment of patients with unresectable malignant pleural mesothelioma. This is the first positive randomized trial of dual immunotherapy in the first line treatment of patients with mesothelioma said the.
A phase 3 trial evaluated nivolumab in combination with ipilimumab in patients with previously untreated mpm and successfully met its primary endpoint of overall survival. According to early results 44 percent of patients cancer did not get worse after taking nivolumab and 50 percent of patients cancer did not get. Nivolumab ipilimumab show positive results for the treatment of malignant pleural mesothelioma.
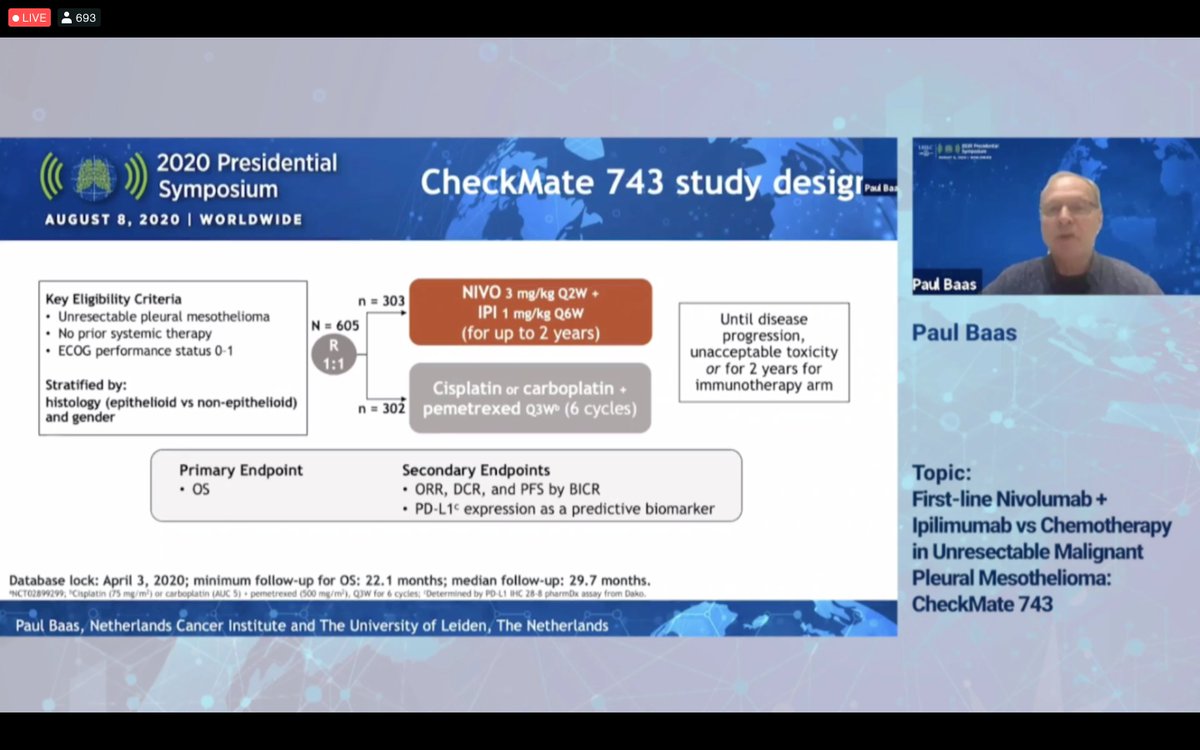
In this single centre phase 2 trial the combination of nivolumab plus ipilimumab showed marked efficacy in patients with recurrent malignant pleural mesothelioma. Nivolumab opdivo in combination with ipilimumab yervoy significantly improved overall survival os in treatment naive patients with unresectable malignant pleural mesothelioma mpm who were treated in the phase 3 checkmate 743 clinical trial nct02899299 bristol myers squibb announced in a press release. 1 results were presented on august 8 during the 2020 world conference on lung.
First line nivolumab plus ipilimumab significantly extended os compared with platinum based chemotherapy for patients with unresectable malignant pleural mesothelioma according to results of an. Our results warrant further investigation of this combination in a phase 3 trial. These regimens require confirmation in larger clinical trials.
Dual checkpoint inhibition with nivolumab and ipilimumab is associated with prolonged overall survival os relative to chemotherapy in treatment naive patients with inoperable malignant pleural mesothelioma show checkmate 743 data. The checkmate 743 data support the potential for nivolumab plus ipilimumab to become a new standard of care histology is a well established prognostic factor in mesothelioma with non epithelioid patients generally experiencing poorer outcomes. The safety profile was consistent with known data on the combination regimen.

Mesothelioma Immunotherapy Combination Gaining Momentum
European Phase Ii Trials Of Nivolumab Plus Ipilimumab Immunotherapy Benefit Pleural Mesothelioma Patients

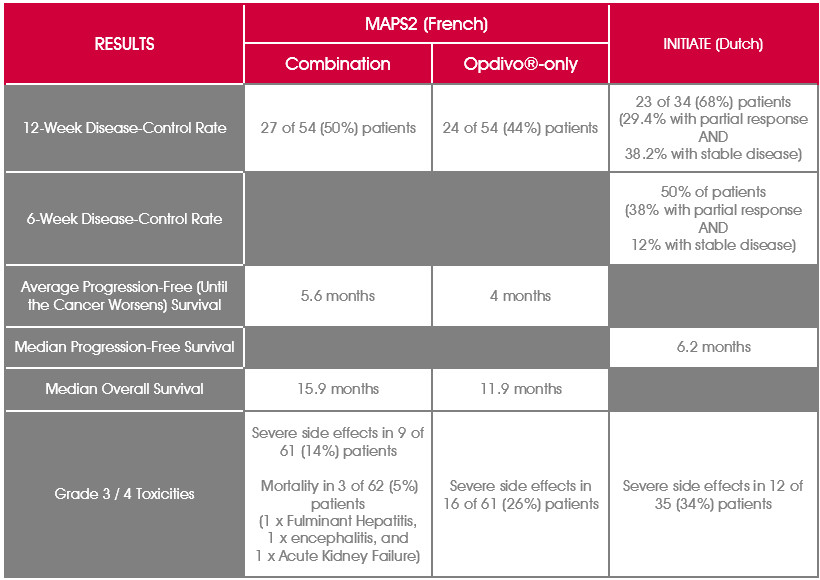
Ipilimumab And Nivolumab In The Treatment Of Recurrent Malignant Pleural Mesothelioma Initiate Results Of A Prospective Single Arm Phase 2 Trial The Lancet Respiratory Medicine

Nivolumab Ipilimumab Demonstrates Durable Os Benefit In Malignant Pleural Mesothelioma Onclive

Ipilimumab And Nivolumab In The Treatment Of Recurrent Malignant Pleural Mesothelioma Initiate Results Of A Prospective Single Arm Phase 2 Trial The Lancet Respiratory Medicine
Https Www Thelancet Com Pdfs Journals Lanonc Piis1470 2045 18 30765 4 Pdf