Providers are looking forward to results from a large phase iii trial that is designed to evaluate nivolumab plus ipilimumab as a first line treatment compared to pemetrexed plus a platinum drug like cisplatin or carboplatin. Nivolumab opdivo in combination with ipilimumab yervoy significantly improved overall survival os in treatment naive patients with unresectable malignant pleural mesothelioma mpm who were treated in the phase 3 checkmate 743 clinical trial nct02899299 bristol myers squibb announced in a press release.
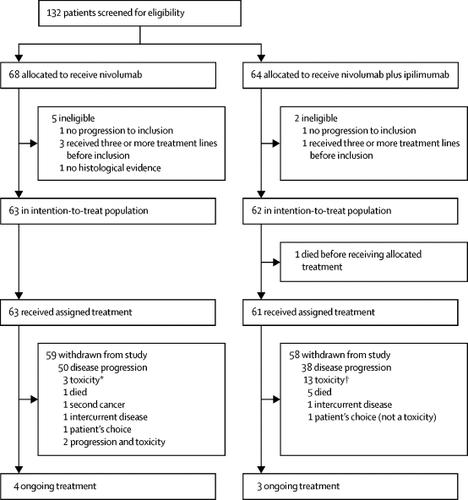
Nivolumab Or Nivolumab Plus Ipilimumab In Patients With Relapsed Malignant Pleural Mesothelioma Ifct 1501 Maps2 A Multicentre Open Label Randomised Non Comparative Phase 2 Trial Lancet Oncol X Mol
Opdivo nivolumab is an immunotherapy drug for advanced non small cell lung cancer.

Mesothelioma nivolumab. In this single centre phase 2 trial the combination of nivolumab plus ipilimumab showed marked efficacy in patients with recurrent malignant pleural mesothelioma. This is called active symptom control. By next fall researchers anticipate to collect more data from the maps 2 trial including more information about mature survival quality of life and biomarker data.
Anti pd 1 nivolumab monotherapy or nivolumab plus anti ctla 4 ipilimumab combination therapy both showed promising activity in relapsed patients with malignant pleural mesothelioma without unexpected toxicity. This is the first positive randomized trial of dual immunotherapy in the first line treatment of patients with mesothelioma said the. Our results warrant further investigation of this combination in a phase 3 trial.
Doctors treat mesothelioma with chemotherapyafter chemotherapy if the mesothelioma comes back the aim is to control symptoms. First line nivolumab plus ipilimumab significantly extended os compared with platinum based chemotherapy for patients with unresectable malignant pleural mesothelioma according to results of an. These regimens require confirmation in larger clinical trials.
Nivolumab is a type of immunotherapy drug called a monoclonal antibodyit works by stimulating the bodys immune system to recognise and kill cancer cells. Researchers want to find out if nivolumab can help these people. Nivolumab plus ipilimumab significantly improved overall survival versus chemotherapy in the first line treatment of patients with unresectable malignant pleural mesothelioma.
Learn about the use of opdivo for those with recurrent mesothelioma. Dual checkpoint inhibition with nivolumab and ipilimumab is associated with prolonged overall survival os relative to chemotherapy in treatment naive patients with inoperable malignant pleural mesothelioma show checkmate 743 data. Researchers hope that nivolumab or the combination of nivolumab with ipilimumab may someday become a proven second line or third line treatment method for pleural mesothelioma.
The safety profile was consistent with known data on the combination regimen. 1 results were presented on august 8 during the 2020 world conference on lung.

Programmed Death 1 Blockade With Nivolumab In Patients With Recurrent Malignant Pleural Mesothelioma Sciencedirect

Paul Baas On First Line Nivolumab Plus Ipilimumab Vs Chemotherapy For Mesothelioma The Asco Post

First Line Nivolumab Ipilimumab Combination Improves Os In Mesothelioma Subset

Sarcomatoid Pleural Mesothelioma Responds To Nivolumab

Nivolumab Mesothelioma Clinical Trials Search For Answers Mesothelioma Circle

Ipilimumab And Nivolumab In The Treatment Of Recurrent Malignant Pleural Mesothelioma Initiate Results Of A Prospective Single Arm Phase 2 Trial The Lancet Respiratory Medicine

Bristol Myers Squibb Releases Optimistic Interim Results In Mesothelioma Clinical Trial Mesothelioma Applied Research Foundation